Automated Calcium Dosing
Calcium is necessary to the growth of various corals as well as other invertebrates. The addition of calcium hydroxide is one popular method of providing a replenished source of calcium to the aquarium system as it is depleted by the inhabitants and calcified deposits.
Three methods are popular for the addition of calcium. The most sophisticated is the use of carbon dioxide (CO2) gas bubbled through a bed of calcium carbonate granules. The carbon dioxide creates carbonic acid that dissolves the calcium carbonate by creating a low pH environment in this type of calcium reactor. The dissolved calcium buffers the solution to create calcium hydroxide (Ca(OH)2). The flow of the carbon dioxide gas must be metered so as not to create a pH that is too low prior to dispensing the solution into the aquarium environment. Pressure regulators are required as is a source of compressed or liquid carbon dioxide. This can become a cumbersome solution.
 Perhaps the most popular method of dosing calcium is through the direct insertion of calcium hydroxide in the form of “kalkwasser”. Kalkwasser is simply a saturated solution of calcium hydroxide. This is added periodically to not only increase the calcium content of the system, but also to increase the pH. Normally reef systems run with pH values in excess of 8.0. Another advantage of increased pH created by calcium hydroxide introduction is the precipitation of various heavy metals and phosphates (algae fertilizer) out of solution. Although this method of calcium introduction is widely used, it is still a manual procedure.
Perhaps the most popular method of dosing calcium is through the direct insertion of calcium hydroxide in the form of “kalkwasser”. Kalkwasser is simply a saturated solution of calcium hydroxide. This is added periodically to not only increase the calcium content of the system, but also to increase the pH. Normally reef systems run with pH values in excess of 8.0. Another advantage of increased pH created by calcium hydroxide introduction is the precipitation of various heavy metals and phosphates (algae fertilizer) out of solution. Although this method of calcium introduction is widely used, it is still a manual procedure.
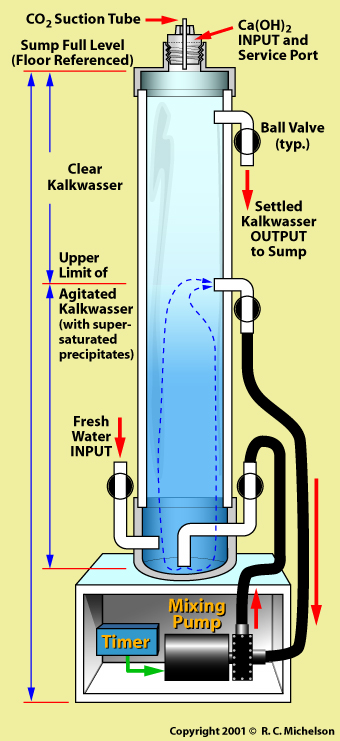
The use of a Nilsen Calcium Reactor is a method of intermittently or continuously dosing calcium hydroxide into the aquarium system through an automation. Basically, as water evaporates from the aquarium, it is replaced by a Kalkwasser solution that is created in a reactor that stores supersaturated calcium hydroxide solution that is not in contact with the atmosphere. It is important that the calcium hydroxide not come in contact with atmospheric carbon dioxide because insoluble calcium carbonate is formed which precipitates out of solution and does not contribute to the maintenance of the pH nor does it provide readily usable calcium to the invertebrates.
When Kalkwasser is mixed, a solution with a very high concentration of Ca++ and OH- ions is created. This solution is very basic (around pH 12). This will react very easily with dissolved CO2 already in the water or coming from the air/water interface. The result is that Ca++ will react with CO2 and create calcium carbonate. This is particularly problematic when systems dose Kalkwasser by dripping it into the aquarium at the surface where interaction with atmospheric CO2 is most likely. In order to keep the pH as high as possible, we desire to put as many OH- ions into solution as possible. Interaction with CO2 locks these OH- ions up as carbonates. Alkalinity is the measure of bicarbonates, carbonates, borates, and hydroxides present in the water. Measures of alkalinity indicate how much acid can be absorbed through the conversion of OH- ions into other products before the pH will drop. The introduction of Kalkwasser not only increases the pH, but buffers the solution to maintain higher values of alkalinity.
Here is what goes on when Kalkwasser is added to the system:
When calcium hydroxide is dissolved in water, we get,
Ca(OH)2 [calcium hydroxide] + H2O [water] -> (Ca++) [calcium ions]+ 2OH- [hydroxide ions] + H2O
In the presence of CO2, the following reaction occurs:
(Ca++) + (2OH-) + CO2 -> (Ca++) + (CO3--) [carbonate ions]+ H2O
A secondary reaction then takes place when the carbonate ions join with the free calcium ions:
(Ca++) + (CO3--) -> CaCO3 [calcium carbonate]
If the amount of CO2 is limited, then the reaction will limit itself to the production of a finite amount of useless calcium carbonate. If CO2 is abundant and replenished, then the calcium carbonate will further combine with the additional carbon dioxide and water to form free calcium ions and bicarbonate ions. While this is a beneficial re-release of calcium, the pH will not increase due to the loss of OH- ions to the bicarbonate, but the alkalinity will be maintained:
CaCO3 + H2O + CO2 -> (Ca++) + 2HCO3- [bicarbonate ions]
The Nilsen reactor is a sealed fresh water column to which calcium hydroxide is periodically added. Internal to this column is a mechanism whereby the solution can be agitated to assure complete mixing, and the creation of a supersaturated calcium hydroxide solution. This solution is then dosed into the aquarium without ever coming in contact with the atmosphere.
Inevitably some carbon dioxide will be present in the water that is used within the Nilsen Calcium Reactor water column. Therefore some precipitation of calcium carbonate will occur. Since this is denser than the calcium hydroxide solution, it will settle to the bottom on the reactor.
 Similarly, to allow the Nilsen Calcium Reactor to function for weeks at a time, it is advisable to overload the column with more calcium hydroxide than can possibly go into a supersaturated solution. This too will settle to the bottom of the reactor. But if the lower portion of the water column is periodically stirred, then this additional calcium hydroxide will be able to mix with newly introduced fresh water to form a continuing supply of supersaturated Kalkwasser.
Similarly, to allow the Nilsen Calcium Reactor to function for weeks at a time, it is advisable to overload the column with more calcium hydroxide than can possibly go into a supersaturated solution. This too will settle to the bottom of the reactor. But if the lower portion of the water column is periodically stirred, then this additional calcium hydroxide will be able to mix with newly introduced fresh water to form a continuing supply of supersaturated Kalkwasser.
It is therefore important to draw water from near the surface of the reactor water column so as not to introduce the calcium carbonate precipitate into the aquarium.
My Nilsen Calcium Reactor design takes “top-off water” that would normally go directly into the aquarium sump to replenish water that has evaporated, and cycles it through the calcium reactor where it is mixed with the stored calcium hydroxide to form a saturated Kalkwasser solution that is then introduced into the aquarium sump. The entire process relies on gravity feed and requires no pumps. A small pump is included in the system to circulate the fresh water with the calcium hydroxide to create the supersaturated Kalkwasser solution, but this is present only for the sake of mixing efficiency since the fresh water is introduced at the bottom on the reactor column where the excess calcium hydroxide has settled.
As an ancillary system, a resistivity sensor and a float sensor have been placed in the base of my Nilsen Calcium Reactor (as shown in the enlargement of my calcium reactor) because it is one of the few aquarium systems that stands directly on the floor of the “aquarium command center” in which the sumps reside. Therefore, were an unseen leak of either fresh water or salt water to occur, one or both of these sensors will activate an alarm which can be heard throughout the house. The resistivity sensor detects the presence of a thin layer of conducting salt water on the floor. The float switch (constructed from a ping pong ball glued to a micro switch) will detect fresh water on the floor (but to a slightly greater depth (approx. 0.5 inches). The “aquarium command center” room is poured concrete with an eight inch lip at the bottom of the entrance door (actually the door is a seven-foot “hatch” through the concrete wall that is raised eight inches above the poured concrete floor), so were a leak to occur, all of the water would be contained in this one room. Therefore the float switch could function were a fresh water pipe to break, since the water would not simply run off to damage another area. All household water enters the house (from the well) and is distributed to the rest of the house from this room. I designed the house and built it to accommodate not only the aquarium, but eventualities such as broken pipes.
Return to Michelson
Aquarium.
 Perhaps the most popular method of dosing calcium is through the direct insertion of calcium hydroxide in the form of “kalkwasser”. Kalkwasser is simply a saturated solution of calcium hydroxide. This is added periodically to not only increase the calcium content of the system, but also to increase the pH. Normally reef systems run with pH values in excess of 8.0. Another advantage of increased pH created by calcium hydroxide introduction is the precipitation of various heavy metals and phosphates (algae fertilizer) out of solution. Although this method of calcium introduction is widely used, it is still a manual procedure.
Perhaps the most popular method of dosing calcium is through the direct insertion of calcium hydroxide in the form of “kalkwasser”. Kalkwasser is simply a saturated solution of calcium hydroxide. This is added periodically to not only increase the calcium content of the system, but also to increase the pH. Normally reef systems run with pH values in excess of 8.0. Another advantage of increased pH created by calcium hydroxide introduction is the precipitation of various heavy metals and phosphates (algae fertilizer) out of solution. Although this method of calcium introduction is widely used, it is still a manual procedure.
